Janssen Canada - Gastroenterology Division
Drug: Stelara® (ustekinumab)
Project: Compliant Website for HCPs, Medical Professionals, Nurses, etc.
Managing Agency: Outpost 379, Canada
- Executed the B2B content strategy for Janssen’s Gastroenterology drug named ‘Stelara®’ that is prescribed to patients/adults suffering from
Moderately to Severely Active Ulcerative Colitis
Moderately to Severely Active Crohn’s Disease
Adults and Children 6 Years And Older with Moderate to Severe Plaque Psoriasis
Adults and Children 6 Years And Older with Active Psoriasis Arthritis
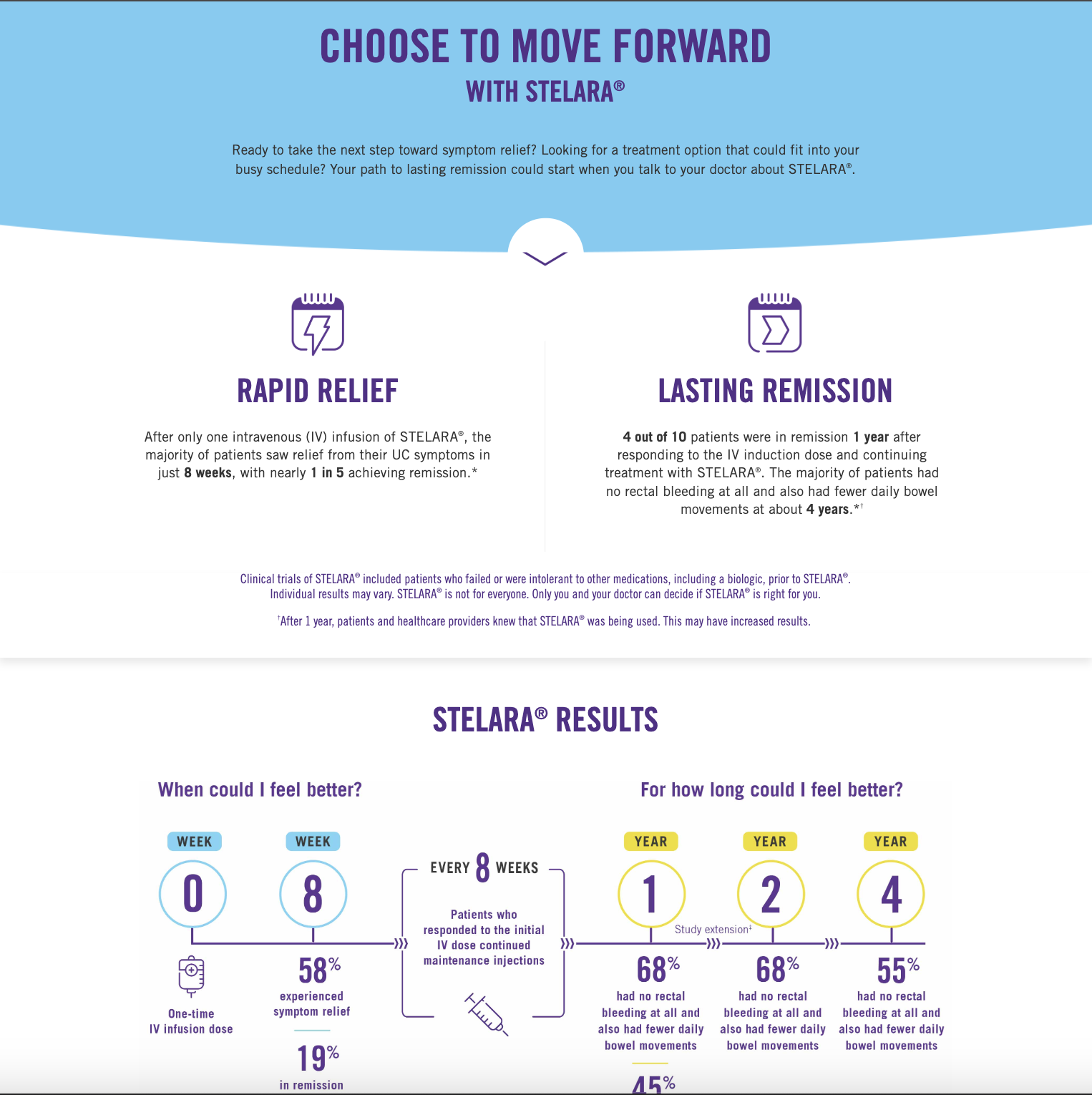
- Contributed to the development of PAAB-approved jnjmedicalpro.ca, a bilingual website (exhibit below) for prescribing medical professionals, HCPs, Care Providers, and patients on Janssen's Stelara®. This initiative improved content accessibility, readability and doubled website traffic within 3 months, while also reducing customer service inquiries by 30% due to the cleaner UX format.
- Assisted in creating diverse print & digital marketing collateral: journal ads, infographics, display ads, emailers/newsletters, product monograph, etc. (exhibit below)
- Executed regulatory compliant projects as per brand guidelines and compliant quality standards.
- Documented campaign activities via Veeva Vault to enhance operational efficiency and supported task delegation for smooth campaign execution.
Key Skills :
Project Management
Creative Production
Launch Planning
Design Direction
Copy Proofreading
Client Coordination
Veeva Vault
The Pharmaceutical Advertising Advisory Board (PAAB) Canada Communications
Key Visual
HCP Collaterals
Including but not limited to:
Dosage Cards
Infographics
Journal Ads
Branded Email Templates
Key Skills:
Legal
Brand Compliant and Regulatory Department Communications
Design / Studio Production
Copy Proofreading
Stakeholder Management
DAM via Veeva Vault